Why do we measure the EC?
Measuring the EC is very quick and easy. We use a simple sensor and within a minute it gives you a hint of the water's quality. We monitor the canal water to see how variable its composition is, and to track the quality of the water that we add to our aquaponics system. We monitor the aquaponics water to see how quickly the salinity of the system changes with the addition of this new water source. Eventually we will be able to see at which EC different plants will show losses in yield.
Other ways to measure salinity is by measuring specific ion concentrations, like sodium (Na) and chloride (Cl). In plant and salinity literature there is no standard paramater for salinity. EC and ion concentrations are both used. For us it is not as easy to measure ion concentrations, but to get an idea we send a sample to the lab every month.
What is EC?
EC stands for electrical conductivity and is a measure of water salinity and water quality. Electrical conductivity measures the ability of water to conduct an electrical current. The higher the concentration of dissolved charged chemicals (also known as salts) in the water, the greater an electrical current can be conducted. Examples of charged ions that naturally occur in water include calcium, potassium, sodium, chloride, sulfate and nitrate (source). Especially sodium and chloride are present at high concentrations in canal water, and contribute the most to the EC. A clear correlation between ion concentrations and EC is hard to establish, since the ion composition in the water is variable and can also differ significantly in different waters.
EC in aquaponics and the canal
The EC of the aquaponics and canal water are both much higher than the EC of tap water. However, for different reasons. The water in aquaponics is very nutrient rich. Potassium, magnesium, calcium, sulfate, and nitrate are present in high concentrations, because they are introduced to the system as fertilizers. All these ions contribute to the EC in aquaponics. However, in this context a high EC is a good thing, because these ‘salts’ are useful building blocks for plants.
In the canal the ions that contribute mostly to the EC are sodium and chloride. These ions negatively affect plant growth at high concentrations. So a high EC in the canal means that the water is not of the best quality for irrigation purposes. What we can learn from this is that EC strongly depends on the composition of different waters, and that it is not a direct measure for the water quality for irrigation purposes. However, keeping the source of the water in mind, it does give a good indication.
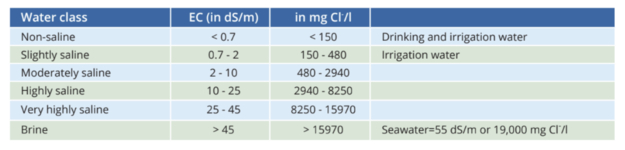
We found that the EC of Het Oosterdok ranges from 1-5.5 mS/cm, and that there is a seasonal fluctuation. The EC is generally lower in the winter time than in the summer time. There is also a correlation with weather conditions like wind and rain. Lastly there are unpredictable changes in EC caused by the opening and closing of the locks of the Noordzeekanaal. Most of the times the water of het Oosterdok is classified as moderately saline (see the figure below).
The relation between EC and Temperature
Another thing we need to consider when using the EC as a salinity measure, is that it depends on temperature. The higher the water temperature, the higher the EC. This dependency is caused by the fact that the ions move faster at higher temperatures, which makes it easier for the electrical current to flow. The water temperature in both the canal and in aquaponics is quite variable, so when we measure the EC we need to correct the value to a standard temperature of 25 degrees.